Enroflox®
(enrofloxacin) Injection for Dogs 2.27%
A fluoroquinolone designed for the management of bacterial diseases with broad-spectrum activity against susceptible species of both Gram-negative and Gram-positive bacteria including those causing dermal, respiratory and urinary tract infections. Each mL of injectable solution contains 22.7 mg of enrofloxacin. Enroflox® Injection is available for dogs only.
Target Species: Dogs
Product Attributes
-
Same active ingredient and dosing regimen as Baytril® (enrofloxacin) Antibacterial Injectable Solution 2.27%
-
Concentration dependent and bactericidal
-
Kills a broad range of susceptible Gram (+) and Gram (-) bacteria
-
Significant savings and improved clinic profit potential versus Baytril® Injectable Solution 2.27%
-
Available in 20 mL, 50 mL and economical 100 mL vials to fit any practice
CAUTION: Federal (U.S.A.) law restricts this drug to use by or on the order of a licensed veterinarian.

CONTRAINDICATIONS: Enrofloxacin is contraindicated in dogs known to be hypersensitive to quinolones. The safe use of enrofloxacin has not been established in large and giant breeds during the rapid growth phase. The use of enrofloxacin is contraindicated in small and medium breed dogs during the rapid growth phase (between 2 and 8 months of age). WARNINGS: For use in animals only. The use of this product in cats may result in retinal toxicity. Keep out of reach of children. Observe label directions and see product labeling for full product information.
-
Active Ingredient(s)
enrofloxacin
-
Dosage Form
Injectable solution
-
Indications
Enroflox® (enrofloxacin) Injection for Dogs 2.27% is indicated for the management of diseases in dogs associated with bacteria susceptible to enrofloxacin.
-
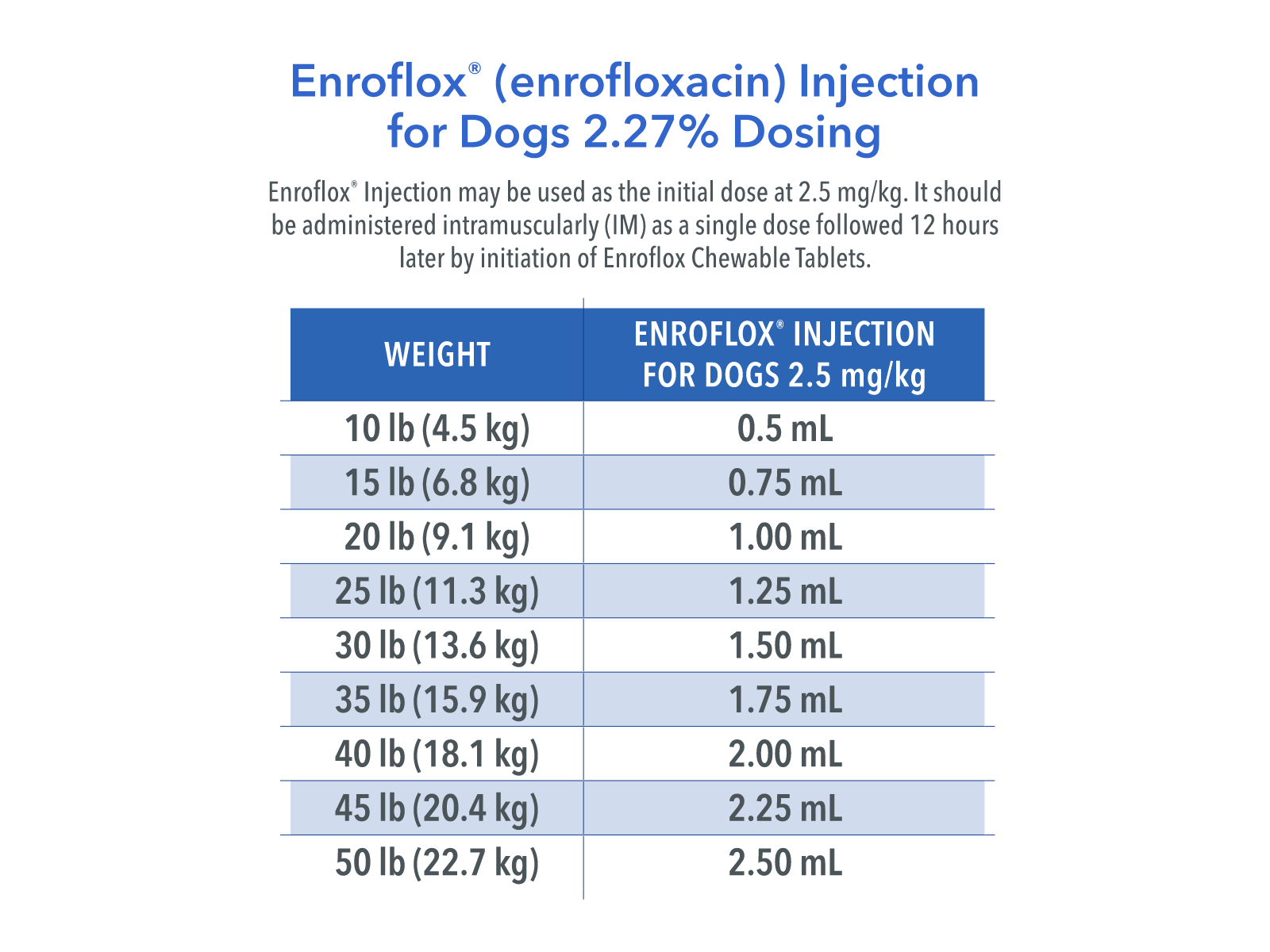
Dosage and Administration
Enroflox® Injection for Dogs may be used as the initial dose at 2.5 mg/kg. It should be administered intramuscularly (IM) as a single dose, followed by initiation of enrofloxacin tablet therapy.
Enroflox® Injection for Dogs may be administered as follows:
Weight of Animal
Enroflox® Injection for Dogs* 2.5 mg/kg
9.1 kg (20 lb)
1.00 mL
27.2 kg (60 lb)
3.00 mL
*The initial Enroflox® Injection for Dogs administration should be followed 12 hours later by initiation of enrofloxacin tablet therapy.
The lower limit of the dose range was based on efficacy studies in dogs where enrofloxacin was administered at 2.5 mg/kg twice daily. Target animal safety and toxicology studies were used to establish the upper limit of the dose range and treatment duration.
-
Storage
Store at 59°–77°F (15° – 25°C). Excursions permitted up to 86°F (30°C). Brief exposure to temperature up to 104°F (40°C) may be tolerated provided the mean kinetic temperature does not exceed 77°F (25°C); however, such exposure should be minimized. Protect from direct sunlight. Do not freeze. Use within 90 days of first puncture.
© 2023 Norbrook Laboratories Limited. The Norbrook logo and Enroflox are registered trademarks of Norbrook Laboratories Limited. Baytril is a registered trademark of Elanco or its affiliates.

Prefer to speak directly to a member of our team?
Norbrook®, Inc.
We are open Mon - Fri 8:00am - 5:00pm CST
Please note: Product information presented on this website is intended only as a brief summary of Norbrook products for your convenience. Not all products or indications are licensed in every country and may be subject to further local variations. For specific product information you should always consult a healthcare professional from your region or visit the local government agency website for the most up to date information. Please see our terms and conditions for further information.