Flunixin Injection
(flunixin meglumine injection)
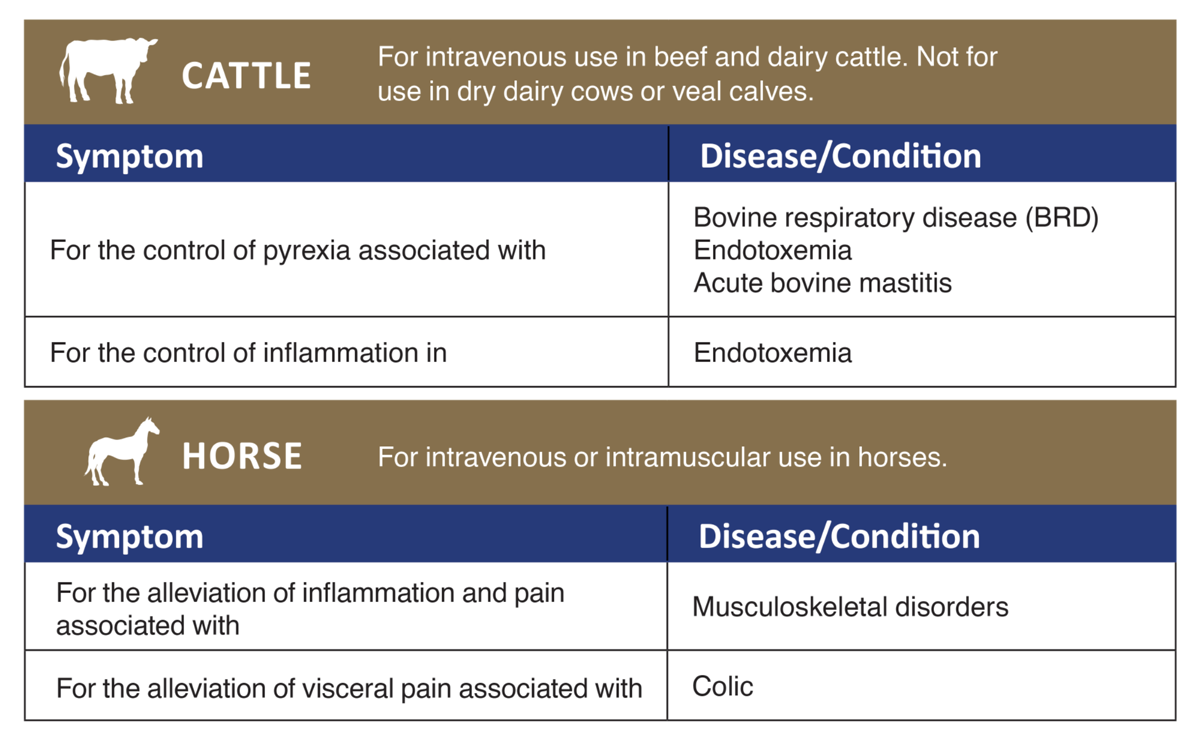
A potent, non-narcotic, non-steroidal, analgesic drug with anti-inflammatory and anti-pyretic activity. It is used to control pyrexia associated with bovine respiratory disease as well as endotoxemia and acute bovine mastitis. It also alleviates musculoskeletal inflammation and pain in horses, including visceral pain associated with colic.
Target Species: Beef cattle, Dairy cattle and Horses
Product Attributes
-
Potent, non-narcotic, non-steroidal analgesic
-
Fast-acting anti-inflammatory and anti-pyretic activity
-
Flunixin Injection is administered intravenously in beef and dairy cattle, and administered intravenously (IV) or intramuscularly (IM) in horses
-
FDA approved
-
Available in 100 mL and 250 mL multi-dose vials
CAUTION: Federal law restricts this drug to use by or on the order of a licensed veterinarian.
Observe label directions and withdrawal times. Not for use in bulls intended for breeding, dry cows or in calves to be processed for veal. Cyclooxygenase inhibitory NSAIDs may be associated with gastrointestinal, renal and hepatic toxicity. Concomitant use with other anti-inflammatory drugs should be avoided or closely monitored. Rare instances of anaphylactic-like reactions, some of which have been fatal, have been reported. See product labeling for full product information.
-
Active Ingredient(s)
flunixin 50 mg/mL
-
Dosage Form
Solution for injection
-
Indications
Horse: Flunixin Injection is recommended for the alleviation of inflammation and pain associated with musculoskeletal disorders in the horse. It is also recommended for the alleviation of visceral pain associated with colic in the horse.
Cattle: Flunixin Injection is indicated for the control of pyrexia associated with bovine respiratory disease, endotoxemia and acute bovine mastitis. Flunixin Injection is also indicated for the control of inflammation in endotoxemia. -
Dosage and Administration
Horse: The recommended dose for musculoskeletal disorders is 0.5 mg per pound (1 mL/100 lbs) of bodyweight once daily. Treatment may be given by intravenous or intramuscular injection and repeated for up to five days. Studies show onset of activity is within 2 hours. Peak response occurs between 12 and 16 hours and duration of activity is 24-36 hours.
The recommended dose for the alleviation of pain associated with equine colic is 0.5 mg per pound of bodyweight. Intravenous administration is recommended for prompt relief. Clinical studies show pain is alleviated in less than 15 minutes in many cases. Treatment may be repeated when signs of colic recur. During clinical studies approximately 10% of the horses required one or two additional treatments. The cause of the colic should be determined and treated with concomitant therapy.
Cattle: The recommended dose for control of pyrexia associated with bovine respiratory disease and endotoxemia and control of inflammation in endotoxemia is 1.1 to 2.2 mg/kg (0.5 to 1 mg/lb; 1 to 2 mL per 100 lbs) of bodyweight given by slow intravenous administration either once a day as a single dose or divided into two doses administered at 12 hour intervals for up to 3 days. The total daily dose should not exceed 2.2 mg/kg (1.0 mg/lb) of bodyweight. Avoid rapid intravenous administration of the drug. The recommended dose for acute bovine mastitis is 2.2 mg/kg (1.0 mg/lb: 2 mL per 100 lbs) of bodyweight given once by intravenous administration.

-
Storage
Store between 2° and 30°C (36° and 86°F). Use within 60 days of first puncture. When using a draw-off spike or needle with a bore diameter larger than 16-gauge, discard any product remaining in the vial immediately after use.
© 2023 Norbrook Laboratories Limited. The Norbrook logo is a registered trademark of Norbrook Laboratories Limited.

Prefer to speak directly to a member of our team?
Norbrook®, Inc.
We are open Mon - Fri 8:00am - 5:00pm CST
Please note: Product information presented on this website is intended only as a brief summary of Norbrook products for your convenience. Not all products or indications are licensed in every country and may be subject to further local variations. For specific product information you should always consult a healthcare professional from your region or visit the local government agency website for the most up to date information. Please see our terms and conditions for further information.