Norfenicol®
(florfenicol) Injectable Solution
Norfenicol® Injectable Solution is a broad-spectrum, fast-acting antibiotic that contains the same active ingredient and is bioequivalent to Nuflor® (florfenicol) Injectable Solution.
Target Species: Beef cattle, Non-Lactating dairy cattle
Added Benefits:
-
Unique formulation
-
Less viscous and more syringeable than Nuflor®1
-
Shorter subcutaneous withdrawal time than Nuflor®
-
Plastic bottles reduce risk of product loss
Same Benefits as Nuflor®:
-
Reaches minimum inhibitory concentration (MIC) in the lungs within 30 minutes2
-
Treats and controls BRD, targeting all three major bacteria that cause BRD*
-
Treats foot rot
-
Labeled for subcutaneous use in cattle at high risk for BRD
-
FDA approved
-
Available in 100 mL, 250 mL and 500 mL plastic multiple-dose vials
1 Data on file.
2 Varma, KJ, Lockwood PW, Cosgrove MS, Rogers ER, Pharmacology, Safety and Clinical Efficacy of Nuflor (florfenicol) Following Subcutaneous Administration to Cattle. Proceedings of a Symposium Held in Conjunction with the XX World Buiatrics Congress. Sydney, Australia. July 1998: 3-19.
*Mannheimia haemolytica, Histophilus somni and Pasteurella multocida
CAUTION: Federal law restricts this drug to use by or on the order of a licensed veterinarian.
Observe label directions and withdrawal times. For use in beef and non-lactating dairy cattle only. Not approved for use in female dairy cattle 20 months of age or older, including dry dairy cows. Animals intended for human consumption must not be slaughtered within 28 days of the last intramuscular treatment or within 33 days of subcutaneous treatment. Do not use in calves to be processed for veal. Intramuscular injection may result in local tissue reaction which may result in trim loss at slaughter. See product labeling for full product information, including adverse reactions.
-
Active Ingredient(s)
florfenicol
-
Dosage Form and Description
Light yellow to straw-colored solution for injection
-
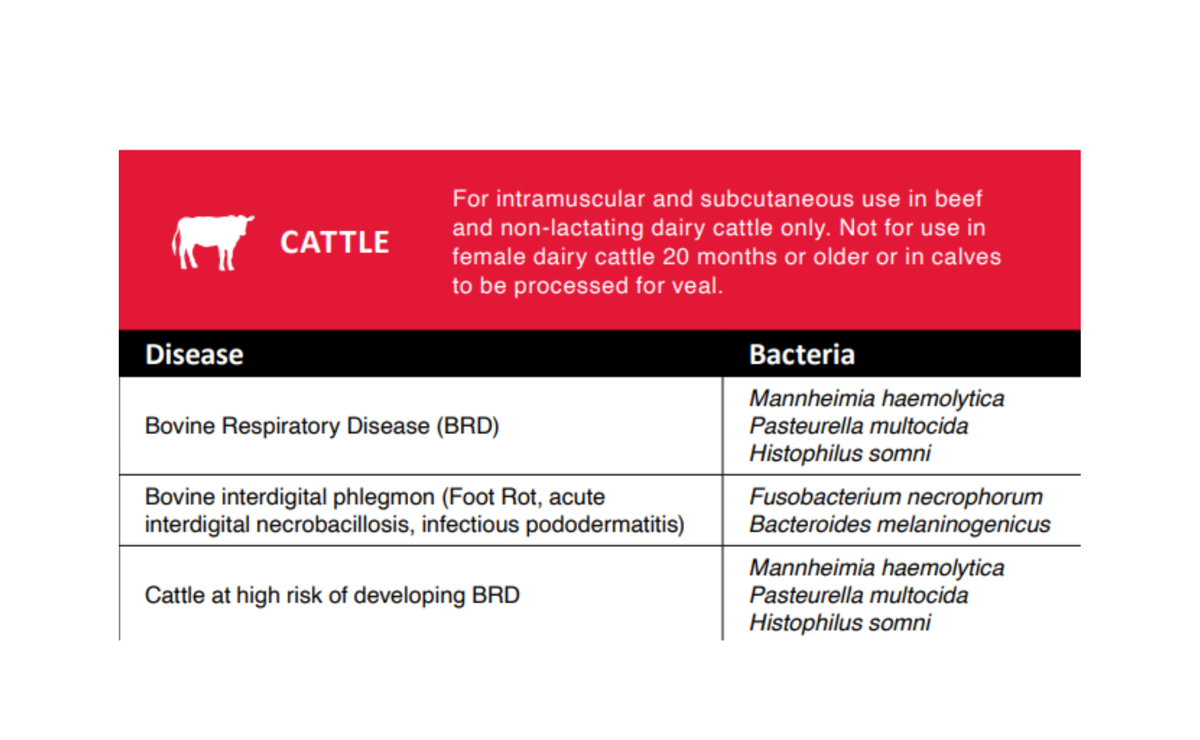
Indications
Norfenicol® Injectable Solution is indicated for treatment of bovine respiratory disease (BRD) associated with Mannheimia haemolytica, Pasteurella multocida, and Histophilus somni, and for the treatment of bovine interdigital phlegmon (foot rot, acute interdigital necrobacillosis, infectious pododermatitis) associated with Fusobacterium necrophorum and Bacteroides melaninogenicus. Also, it is indicated for the control of respiratory disease in cattle at high risk of developing BRD associated with Mannheimia haemolytica, Pasteurella multocida, and Histophilus somni.
-
Dosage and Administration
For treatment of bovine respiratory disease (BRD) and bovine interdigital phlegmon (foot rot): Norfenicol® Injectable Solution should be administered by intramuscular injection to cattle at a dose rate of 20 mg/kg body weight (3 mL/100 lbs). A second dose should be administered 48 hours later. Alternatively, Norfenicol® Injectable Solution can be administered by a single subcutaneous (SC) injection to cattle at a dose rate of 40 mg/kg body weight (6 mL/100 lbs). Do not administer more than 10 ml at each site. The injection should be given only in the neck.
NOTE: Intramuscular injection may result in local tissue reaction which persists beyond 28 days. This may result in trim loss of edible tissue at slaughter. Tissue reaction at injection sites other than the neck is likely to be more severe.For control of respiratory disease in cattle at high-risk of developing BRD: Norfenicol® Injectable Solution should be administered by a single subcutaneous injection to cattle at a dose rate of 40 mg/kg body weight (6 mL/100 lbs). Do not administer more than 10 mL at each site. The injection should be given only in the neck.

Clinical improvement should be evident in most treated subjects within 24 hours of initiation of treatment. If a positive response is not noted within 72 hours of initiation of treatment, the diagnosis should be re-evaluated.

-
Storage
Store at or below 77°F (25°C). Refrigeration is not required. Excursions permitted up to 86°F (30°C). Brief exposure to temperature up to 104°F (40°C) may be tolerated provided the mean kinetic temperature does not exceed 77°F (25°C); however, such exposure should be minimized. The solution is light yellow to straw colored. Color does not affect potency. Use within 28 days of first vial puncture.
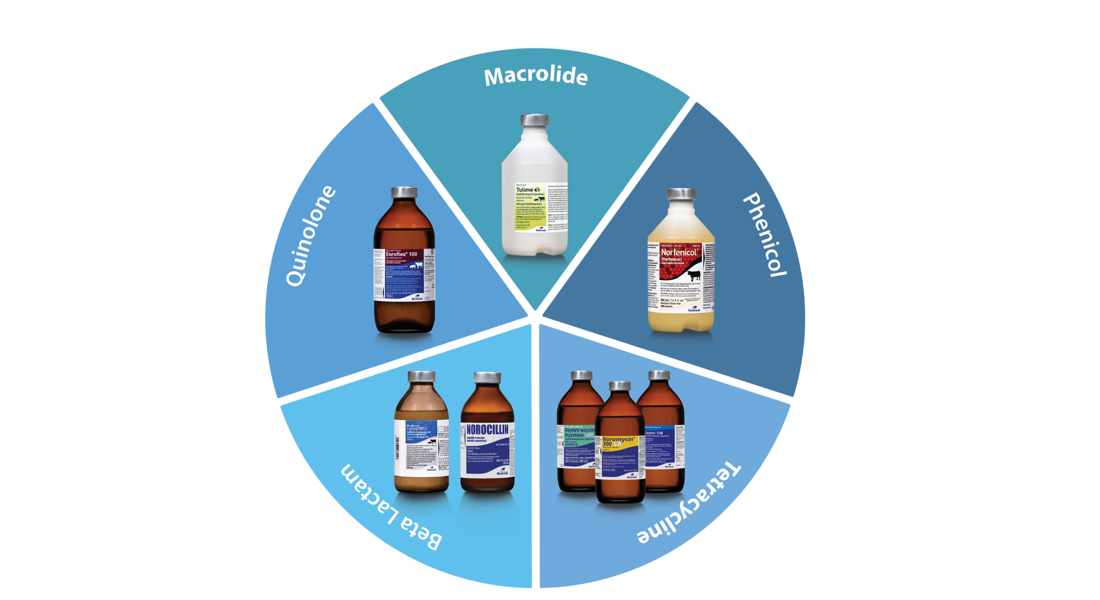
A Comprehensive Antibiotic Portfolio
Norbrook® now covers five classes of antibiotics.
© 2023 Norbrook Laboratories Limited. The Norbrook logo and Norfenicol are registered trademarks of Norbrook Laboratories Limited. Nuflor is a registered trademark of Merck Animal Health.

Prefer to speak directly to a member of our team?
Norbrook®, Inc.
We are open Mon - Fri 8:00am - 5:00pm CST
Please note: Product information presented on this website is intended only as a brief summary of Norbrook products for your convenience. Not all products or indications are licensed in every country and may be subject to further local variations. For specific product information you should always consult a healthcare professional from your region or visit the local government agency website for the most up to date information. Please see our terms and conditions for further information.