Oxytetracycline Injection 200
(oxytetracycline injection)
Broad-spectrum antibiotic for use in beef cattle, dairy cattle, calves (including preruminating veal calves) and swine.
Target Species: Beef cattle, Dairy Cattle (including preruminating veal calves) and Swine
Product Attributes
-
Convenient to use: In cattle, administer either subcutaneously (SQ), intramuscularly (IM) or intravenously (IV) for specific needs and duration; in swine, administer intramuscularly only
-
Broad-spectrum, ready-to-use antibiotic
-
FDA approved
-
Available in 100 mL, 250 mL and 500 mL vials
CAUTION: Federal law restricts this drug to use by or on the order of a licensed veterinarian.
Observe label directions and withdrawal times. Adverse reactions, including injection site swelling, restlessness, ataxia, trembling, swelling of the eyelids, ears, muzzle, anus and vulva (or scrotum and sheath in males), respiratory abnormalities (labored breathing), frothing at the mouth, collapse and possibly death have been reported. See product labeling for full product information.
-
Active Ingredient(s)
Each mL contains 200 mg of oxytetracycline
-
Dosage Form
Sterile, ready-to-use injection
-
Indications
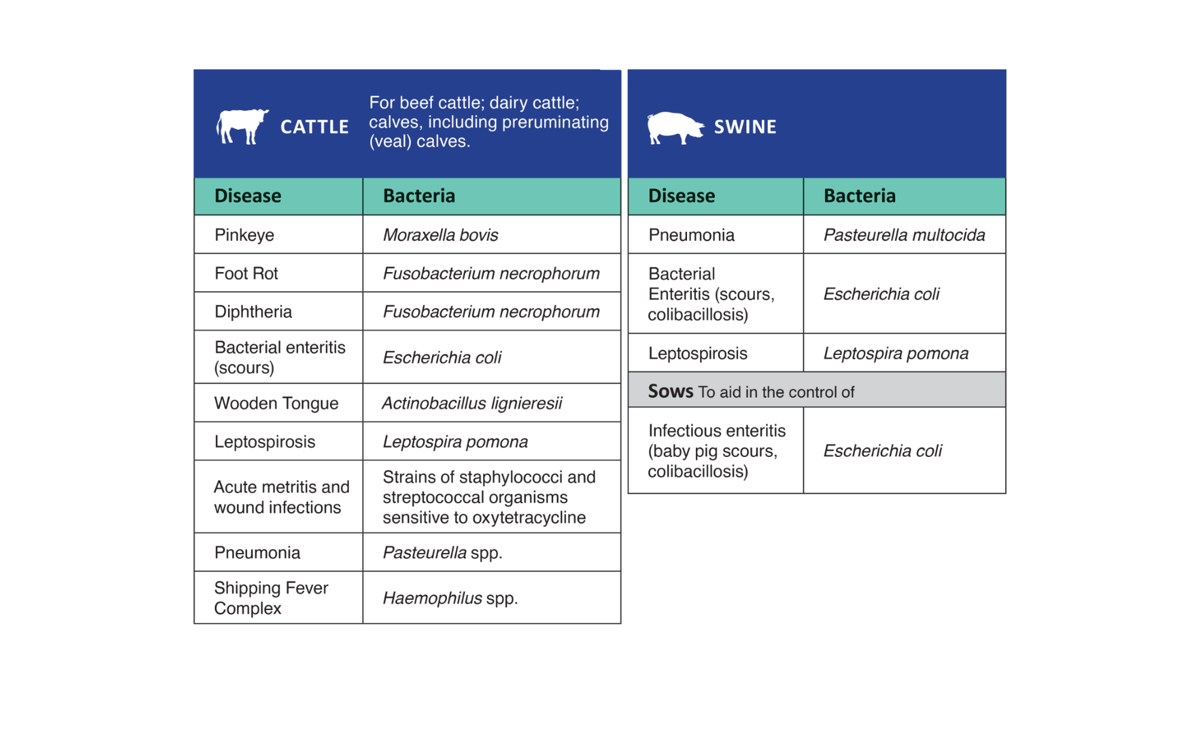
Oxytetracycline Injection 200 is intended for use in the treatment of the following diseases in beef cattle; dairy cattle; calves, including preruminating (veal) calves; and swine when due to oxytetracycline-susceptible organisms:
Cattle: Oxytetracycline Injection 200 is indicated in the treatment of pneumonia and shipping fever complex associated with Pasteurella spp. and Haemophilus spp.; infectious bovine keratoconjunctivitis (pink eye) caused by Moraxella bovis; foot rot and diphtheria caused by Fusobacterium necrophorum; bacterial enteritis (scours) caused by Escherichia coli; wooden tongue caused by Actinobacillus lignieresii; leptospirosis caused by Leptospira pomona; and wound infections and acute metritis caused by strains of staphylococci and streptococci organisms sensitive to oxytetracycline.
Swine: Oxytetracycline Injection 200 is indicated in the treatment of bacterial enteritis (scours, colibacillosis) caused by Escherichia coli; pneumonia caused by Pasteurella multocida; and leptospirosis caused by Leptospira pomona.
In sows, Oxytetracycline Injection 200 is indicated as an aid in the control of infectious enteritis (baby pig scours, colibacillosis) in suckling pigs caused by Escherichia coli.
-
Dosage and Administration
Cattle: Oxytetracycline Injection 200 is to be administered by intramuscular, subcutaneous, or intravenous injection. Intramuscular administration is not recommended according to Beef Quality Assurance Guidelines.
A single dosage of 9 mg of Oxytetracycline Injection 200 per lb of body weight administered intramuscularly or subcutaneously is recommended in the treatment of the following conditions:
(1) bacterial pneumonia caused by Pasteurella spp. (shipping fever) in calves and yearlings, where retreatment is impractical due to husbandry conditions, such as cattle on range, or where repeated restraint is inadvisable.
(2) infectious bovine keratoconjunctivitis (pink eye) caused by Moraxella bovis.
Oxytetracycline Injection 200 can also be administered by intravenous, subcutaneous, or intramuscular injection at a level of 3-5 mg of oxytetracycline per lb of body weight per day. In the treatment of severe foot rot and advanced cases of other indicated diseases, a dosage level of 5 mg/lb of body weight per day is recommended. Treatment should be continued 24-48 hours following remission of disease signs; however, not to exceed a total of 4 consecutive days. Consult your veterinarian if improvement is not noted within 24-48 hours of the beginning of treatment.
Swine: A single dosage of 9 mg of Oxytetracycline Injection 200 per lb of body weight administered intramuscularly in the neck region is recommended in the treatment of bacterial pneumonia caused by Pasteurella multocida in swine, where re-treatment is impractical due to husbandry conditions or where repeated restraint is inadvisable.
Oxytetracycline Injection 200 can also be administered by intramuscular injection at a level of 3-5 mg of oxytetracycline per lb of body weight per day. Treatment should be continued 24-48 hours following remission of disease signs; however, not to exceed a total of 4 consecutive days. Consult your veterinarian if improvement is not noted within 24-48 hours of the beginning of treatment.
For sows, administer once intramuscularly in the neck region 3 mg of oxytetracycline per lb of body weight approximately 8 hours before farrowing or immediately after completion of farrowing.
For swine weighing 25 lb of body weight and under, Oxytetracycline Injection 200 should be administered undiluted for treatment at 9 mg/lb but should be administered diluted for treatment at 3 or 5 mg/lb.
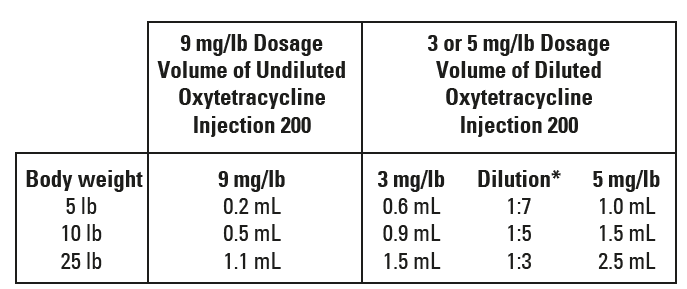
* To prepare dilutions, add one part of Oxytetracycline Injection 200 to 3, 5, or 7 parts of sterile water, or 5% dextrose solution as indicated; the diluted product should be used immediately.

-
Storage
Store at controlled room temperature 20-25°C (68-77°F); excursions permitted 15-30°C (59-86°F). Protect from freezing. For 100 mL size: Use within 60 days of first puncture and puncture a maximum of 36 times. For 250 mL and 500 mL sizes: Use within 60 days for first puncture and puncture a maximum of 36 times. If using a needle or draw-off spike larger than 16 gauge, disregard any remaining product immediately after use.
A Comprehensive Antibiotic Portfolio
Norbrook® now covers five classes of antibiotics.
© 2023 Norbrook Laboratories Limited. The Norbrook logo is a registered trademark of Norbrook Laboratories Limited.

Prefer to speak directly to a member of our team?
Norbrook®, Inc.
We are open Mon - Fri 8:00am - 5:00pm CST
Please note: Product information presented on this website is intended only as a brief summary of Norbrook products for your convenience. Not all products or indications are licensed in every country and may be subject to further local variations. For specific product information you should always consult a healthcare professional from your region or visit the local government agency website for the most up to date information. Please see our terms and conditions for further information.