Foot Rot in Cattle
What is Foot Rot?
Foot rot (necrotic pododermatitis, interdigital necrobacillosis) is a painful, acute, infectious disease of the interdigital tissues in cattle. It is initiated by an insult to the interdigital skin by rocks, dirt clods, frozen mud, ice, rough surfaces, etc., and subsequent invasion by bacterial pathogens. Fusobacterium necrophorum and Bacteroides melaninogenicus are two common bacteria that use their toxins to damage the superficial and deep tissues between the claws. Incidence of footrot may increase during wet conditions when tissues are more soft and susceptible, although the disease can occur at any time.
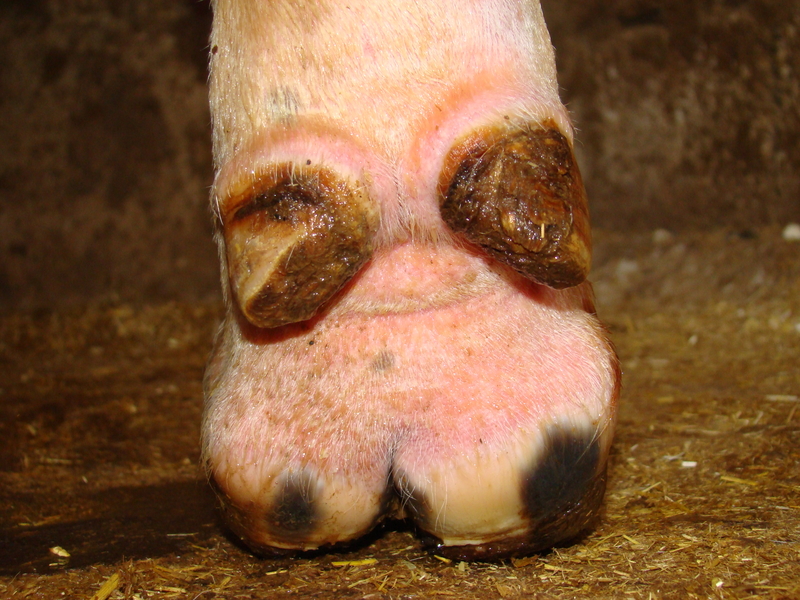


Economic Impact of Foot Rot
The economic impact of foot rot stems from lost production (average daily gain, milk production, fertility, etc.) in addition to associated treatment and labor costs.
Understanding Foot Rot
Learn more about the disease, causes, signs, treatment options and how to control foot rot.
Safety Information
Cefenil® RTU (ceftiofur hydrochloride sterile suspension)
Observe label directions and withdrawal times. Not for use in calves to be processed for veal. As with all drugs, the use of Cefenil® RTU (ceftiofur hydrochloride sterile suspension) is contraindicated in animals previously found to be hypersensitive to the drug. Download product labeling here for full product information.
Norfenicol® (florfenicol) Injectable Solution
CAUTION: Federal law restricts this drug to use by or on the order of a licensed veterinarian. Observe label directions and withdrawal times. For use in beef and non-lactating dairy cattle only. Not approved for use in female dairy cattle 20 months of age or older, including dry dairy cows. Animals intended for human consumption must not be slaughtered within 28 days of the last intramuscular treatment or within 33 days of subcutaneous treatment. Do not use in calves to be processed for veal. Intramuscular injection may result in local tissue reaction which may result in trim loss at slaughter. Download product labeling here for full product information, including adverse reactions.
Noromycin® 300 LA (oxytetracycline injection)
CAUTION: Federal law restricts this drug to use by or on the order of a licensed veterinarian. Observe label directions and withdrawal times. Not for use in lactating dairy animals. Adverse reactions, including injection site swelling, restlessness, ataxia, trembling, respiratory abnormalities (labored breathing), collapse and possibly death have been reported. Download product labeling here for full product information.
Oxytetracycline Injection 200 (oxytetracycline injection)
CAUTION: Federal law restricts this drug to use by or on the order of a licensed veterinarian. Observe label directions and withdrawal times. Adverse reactions, including injection site swelling, restlessness, ataxia, trembling, swelling of the eyelids, ears, muzzle, anus and vulva (or scrotum and sheath in males), respiratory abnormalities (labored breathing), frothing at the mouth, collapse and possibly death have been reported. See product labeling here for full product information.
Tulieve® (tulathromycin injection) Injectable Solution
CAUTION: Federal (USA) law restricts this drug use by or on the order of a licensed veterinarian. IMPORTANT SAFETY INFORMATION FOR CATTLE: Do not use in female dairy cattle 20 months of age or older, including dry dairy cows. Effects on reproductive performance, pregnancy, and lactation have not been determined. Tulieve® has a pre-slaughter withdrawal time of 18 days. Tulieve® should not be used in animals known to be hypersensitive to the product. See product labeling here for full product information.
The Norbrook logo, Cefenil, Norfenicol, Noromycin and Tulieve are registered trademarks of Norbrook Laboratories Limited.
005-25-129