Loxicom 1.5mg/ml Oral Suspension Dogs
Loxicom Oral for dogs is an oral suspension for dogs contains 1.5mg of meloxicam. It is indicated for the alleviation of inflammation and pain in both acute and chronic musculo-skeletal disorders in dogs.
Target Species: Dogs
-
Active Ingredient
Each ml contains: Active Substance: Meloxicam 1.5 mg Excipients: Sodium Benzoate 1.5 mg
-
Pharmaceutical Form
Pale yellow oral suspension.
-
Indications
Alleviation of inflammation and pain in both acute and chronic musculo-skeletal disorders in dogs.
-
Dosage
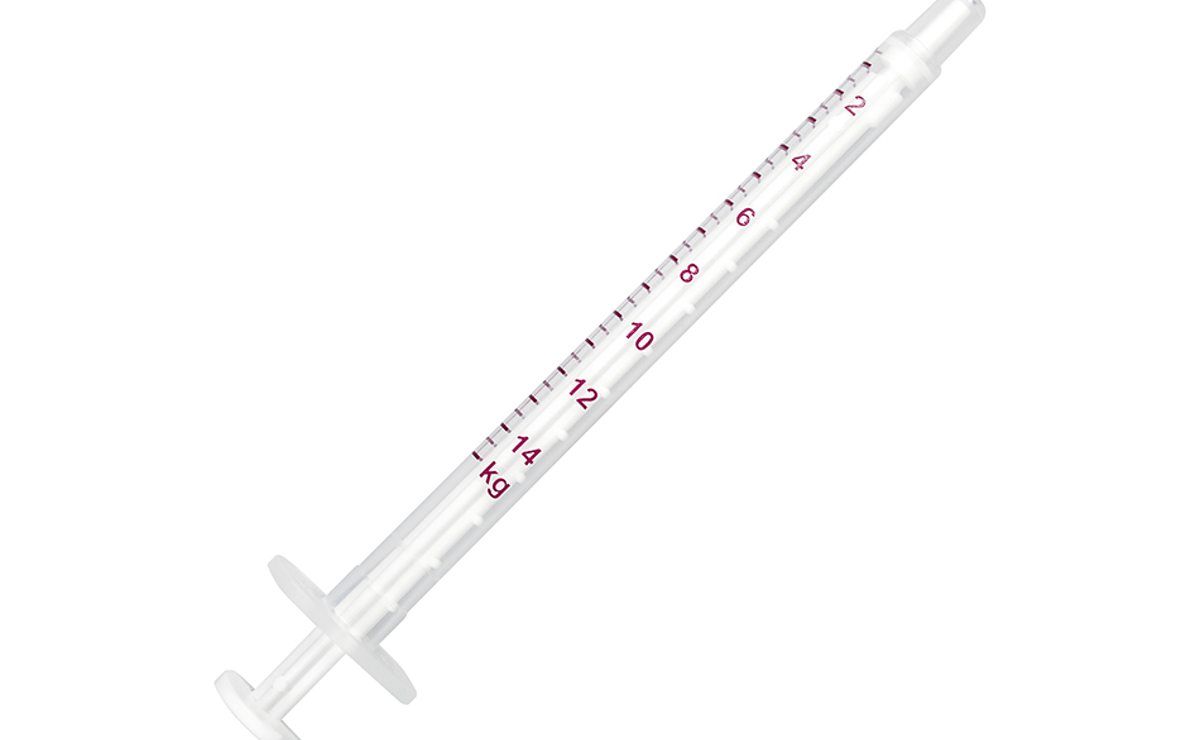
Oral use. To be administered with food or directly into the mouth. Shake well before use. Initial treatment is a single dose of 0.2 mg meloxicam/kg bodyweight (i.e. 1.33 ml/10 kg bodyweight) on the first day. Treatment is to be continued once daily by oral administration (at 24 hour intervals) at a maintenance dose of 0.1 mg meloxicam/kg bodyweight (i.e. 0.667 ml/10 kg bodyweight). For longer term treatment, once clinical response has been observed (after 4 days), the dose can be adjusted to the lowest effective individual dose reflecting that the degree of pain and inflammation associated with chronic musculo-skeletal disorders may vary over time. Particular care should be taken with regard to the accuracy of dosing. The suspension can be given using either of the two measuring syringes provided in the package (depending on weight of dog). The syringes fit onto the bottle and have a kg-bodyweight scale which corresponds to the maintenance dose (i.e. 0.1 mg meloxicam/kg bodyweight). Thus for the first day, twice the maintenance volume will be required. Alternatively therapy may be initiated with Loxicom 5 mg/ml solution for injection. A clinical response is normally seen within 3-4 days. Treatment should be discontinued after 10 days at the latest if no clinical improvement is apparent. Avoid introduction of contamination during use.

Prefer to speak directly to a member of our team?
Norbrook GB
We are open. Mon - Fri 9:00am - 5:00pm
Latest GB News & Articles
Please note: Product information presented on this website is intended only as a brief summary of Norbrook products for your convenience. Not all products or indications are licensed in every country and may be subject to further local variations. For specific product information you should always consult a healthcare professional from your region or visit the local government agency website for the most up to date information. Please see our terms and conditions for further information.